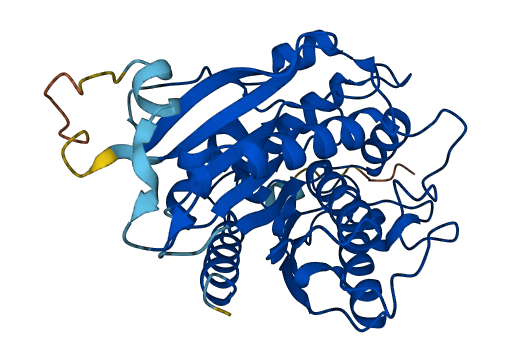
- Information
- Symbol: GID1,OsGID1
- MSU: LOC_Os05g33730
- RAPdb: Os05g0407500
- PSP score
- LOC_Os05g33730.1: 0.0055
- PLAAC score
- LOC_Os05g33730.1: 0
- pLDDT score
- 92.66
- Protein Structure from AlphaFold and UniProt
- MolPhase score
- LOC_Os05g33730.1: 0.00246601
- MolPhase Result
- Publication
- Thermodynamic characterization of OsGID1-gibberellin binding using calorimetry and docking simulations, 2011, J Mol Recognit.
- Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, 2010, Plant Cell.
- Gibberellin homeostasis and plant height control by EUI and a role for gibberellin in root gravity responses in rice, 2008, Cell Res.
- gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, 2006, Plant, Cell and Environment.
- The suppressive function of the rice DELLA protein SLR1 is dependent on its transcriptional activation activity, 2012, Plant J.
- GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin, 2005, Nature.
- Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin, 2007, Plant Cell.
- Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, 2008, Plant Cell.
- A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins, 2010, Plant Cell.
- GID1 modulates stomatal response and submergence tolerance involving ABA and GA signaling in rice., 2014, J Integr Plant Biol.
- Genbank accession number
- Key message
- To investigate gibberellin (GA) signaling using the rice (Oryza sativa) GA receptor GIBBERELLIN-INSENSITIVE DWARF1 (GID1) mutant gid1-8, we isolated a suppressor mutant, Suppressor of gid1-1 (Sgd-1)
- GA perception by GID1 causes SLR1 protein degradation involving the F-box protein GID2; this triggers GA-associated responses such as shoot elongation and seed germination
- Thermodynamic characterization of OsGID1-gibberellin binding using calorimetry and docking simulations
- An exception is the GA-insensitive F-box mutant gid2, which shows milder dwarfism than mutants such as gid1 and cps even though it accumulates higher levels of SLR1
- The level of SLR1 protein in gid2 was decreased by loss of GID1 function or treatment with a GA biosynthesis inhibitor, and dwarfism was enhanced
- Conversely, overproduction of GID1 or treatment with GA(3) increased the SLR1 level in gid2 and reduced dwarfism
- Disturbing GA homeostasis affected the expression of the GA signaling genes GID1 (GIBBERELLIN INSENSITIVE DWARF 1), GID2 and SLR1
- Previously, a yeast two-hybrid (Y2H) assay revealed that the GID1-GA complex directly interacts with SLENDER RICE1 (SLR1), a DELLA repressor protein in GA signaling
- GA(4) was found to have the highest affinity to GID1 in Y2H assays and is the most effective form of GA in planta
- To identify the important regions of GID1 for GA and SLR1 interactions, we used many different mutant versions of GID1, such as the spontaneous mutant GID1s, N- and C-terminal truncated GID1s, and mutagenized GID1 proteins with conserved amino acids replaced with Ala
- The amino acid residues important for SLR1 interaction completely overlapped the residues required for GA binding that were scattered throughout the GID1 molecule
- GIBBERELLIN INSENSITIVE DWARF1 (GID1) encodes a soluble gibberellin (GA) receptor that shares sequence similarity with a hormone-sensitive lipase (HSL)
- It is assumed that interaction between GIBBERELLIN INSENSITIVE DWARF1 (GID1) and the N-terminal DELLA/TVHYNP motif of SLR1 triggers F-box protein GID2-mediated SLR1 degradation
- Under normal growth conditions, there was more PBZ1 protein in gid1 than in the wild type
- A recessive gibberellin (GA)-insensitive dwarf mutant of rice, gibberellin-insensitive dwarf1 (gid1), has been identified, which shows a severe dwarf phenotype and contains high concentrations of endogenous GA
- gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein (PBZ1) in response to cold stress and pathogen attack
- The entcopalyl diphosphate synthase (OsCPS) genes, which encode enzymes at the branch point between GA and phytoalexin biosynthesis, were expressed differentially in gid1 relative to the wild type
- Specifically, OsCPS1, which encodes an enzyme in the GA biosynthesis pathway, was down-regulated and OsCPS2 and OsCPS4, which encode enzymes in phytoalexin biosynthesis, were up-regulated in gid1
- These results suggest that the expression of PBZ1 is regulated by GA signalling and stress stimuli, and that gid1 is involved in tolerance to cold stress and resistance to blast fungus
- When the gibberellin (GA) receptor GIBBERELLIN INSENSITIVE DWARF 1 (GID1) binds to GA, GID1 interacts with DELLA proteins, repressors of GA signaling
- Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin
- In addition, gid1 showed increased tolerance to cold stress and resistance to blast fungus infection
- Together, our results indicate that GID1 is a soluble receptor mediating GA signalling in rice
- A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins
- This activity was suppressed by the GA-dependent GID1-SLR1 interaction, which may explain why GA responses are induced in the presence of GA
- Our results indicate that the N-terminal region of SLR1 has two roles in GA signaling: interaction with GID1 and transactivation activity
- These results indicate that derepression of SLR1 repressive activity can be accomplished by GA and GID1 alone and does not require F-box (GID2) function
- Evidence for GA signaling without GID2 was also provided by the expression behavior of GA-regulated genes such as GA-20oxidase1, GID1, and SLR1 in the gid2 mutant
- Here we report the isolation and characterization of a new GA-insensitive dwarf mutant of rice, gid1
- In rice, the initial GA-signaling events involve the binding of a GA to the soluble GA receptor protein, GID1
- Herein, we present a study aimed at defining the types of forces important to binding via a combination of isothermal titration calorimetry (ITC) and computational docking studies that employed rice GID1 (OsGID1), OsGID1 mutants, which were designed to have a decreased possible number of hydrogen bonds with bound GA, and GA variants
- We find that, in general, GA binding is enthalpically driven and that a hydrogen bond between the phenolic hydroxyl of OsGID1 Tyr134 and the C-3 hydroxyl of a GA is a defining structural element
- GID1 modulates stomatal response and submergence tolerance involving ABA and GA signaling in rice.
- Interestingly, the gid1 mutant had increased levels of chlorophyll and carbohydrates under submergence condition, and showed enhanced ROS-scavenging ability and submergence tolerance compared with the wild-type
- Further analyses suggested that the function of GID1 in submergence responses is partially dependent on ABA, and GA signaling by GID1 is involved in submergence tolerance by modulating carbohydrate consumption
- Taken together, these findings suggest GID1 plays distinct roles in stomatal response and submergence tolerance through both the ABA and GA signaling pathways in rice
- The gid1 mutant showed impaired biosynthesis of endogenous ABA under drought stress condition, but it exhibited enhanced sensitivity to exogenous ABA
- Scanning electron microscope and infrared thermal image analysis indicated an increase in the stomatal conductance in the gid1 mutant under drought condition
- Connection
- GID1~OsGID1, SLR1~OsGAI, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, It is assumed that interaction between GIBBERELLIN INSENSITIVE DWARF1 (GID1) and the N-terminal DELLA/TVHYNP motif of SLR1 triggers F-box protein GID2-mediated SLR1 degradation
- GID1~OsGID1, SLR1~OsGAI, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, The GA-dependent degradation of SLR1(G576V) was reduced in Slr1-d4, and compared with SLR1, SLR1(G576V) showed reduced interaction with GID1 and almost none with GID2 when tested in yeast cells
- GID1~OsGID1, SLR1~OsGAI, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, Surface plasmon resonance of GID1-SLR1 and GID1-SLR1(G576V) interactions revealed that the GRAS domain of SLR1 functions to stabilize the GID1-SLR1 interaction by reducing its dissociation rate and that the G576V substitution in SLR1 diminishes this stability
- GID1~OsGID1, SLR1~OsGAI, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, These results suggest that the stable interaction of GID1-SLR1 through the GRAS domain is essential for the recognition of SLR1 by GID2
- GID1~OsGID1, SLR1~OsGAI, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, We propose that when the DELLA/TVHYNP motif of SLR1 binds with GID1, it enables the GRAS domain of SLR1 to interact with GID1 and that the stable GID1-SLR1 complex is efficiently recognized by GID2
- OsGF14e~GF14e~GID2, GID1~OsGID1, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, It is assumed that interaction between GIBBERELLIN INSENSITIVE DWARF1 (GID1) and the N-terminal DELLA/TVHYNP motif of SLR1 triggers F-box protein GID2-mediated SLR1 degradation
- OsGF14e~GF14e~GID2, GID1~OsGID1, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, The GA-dependent degradation of SLR1(G576V) was reduced in Slr1-d4, and compared with SLR1, SLR1(G576V) showed reduced interaction with GID1 and almost none with GID2 when tested in yeast cells
- OsGF14e~GF14e~GID2, GID1~OsGID1, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, These results suggest that the stable interaction of GID1-SLR1 through the GRAS domain is essential for the recognition of SLR1 by GID2
- OsGF14e~GF14e~GID2, GID1~OsGID1, Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice, We propose that when the DELLA/TVHYNP motif of SLR1 binds with GID1, it enables the GRAS domain of SLR1 to interact with GID1 and that the stable GID1-SLR1 complex is efficiently recognized by GID2
- GID1~OsGID1, SLR1~OsGAI, Gibberellin homeostasis and plant height control by EUI and a role for gibberellin in root gravity responses in rice, Disturbing GA homeostasis affected the expression of the GA signaling genes GID1 (GIBBERELLIN INSENSITIVE DWARF 1), GID2 and SLR1
- OsGF14e~GF14e~GID2, GID1~OsGID1, Gibberellin homeostasis and plant height control by EUI and a role for gibberellin in root gravity responses in rice, Disturbing GA homeostasis affected the expression of the GA signaling genes GID1 (GIBBERELLIN INSENSITIVE DWARF 1), GID2 and SLR1
- GID1~OsGID1, OsCPS~OsCPS1, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, The entcopalyl diphosphate synthase (OsCPS) genes, which encode enzymes at the branch point between GA and phytoalexin biosynthesis, were expressed differentially in gid1 relative to the wild type
- GID1~OsGID1, OsCPS~OsCPS1, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, Specifically, OsCPS1, which encodes an enzyme in the GA biosynthesis pathway, was down-regulated and OsCPS2 and OsCPS4, which encode enzymes in phytoalexin biosynthesis, were up-regulated in gid1
- GID1~OsGID1, OsCPS4~OsCyc1, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, Specifically, OsCPS1, which encodes an enzyme in the GA biosynthesis pathway, was down-regulated and OsCPS2 and OsCPS4, which encode enzymes in phytoalexin biosynthesis, were up-regulated in gid1
- GID1~OsGID1, OsPR10a~PBZ1, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, Among the proteins hyper-accumulated in gid1 were osmotin, triosephosphate isomerase, probenazole inducible protein (PBZ1) and pathogenesis-related protein 10
- GID1~OsGID1, OsPR10a~PBZ1, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, Under normal growth conditions, there was more PBZ1 protein in gid1 than in the wild type
- GID1~OsGID1, OsPR10a~PBZ1, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, These results suggest that the expression of PBZ1 is regulated by GA signalling and stress stimuli, and that gid1 is involved in tolerance to cold stress and resistance to blast fungus
- GID1~OsGID1, OsPR10a~PBZ1, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein (PBZ1) in response to cold stress and pathogen attack
- GID1~OsGID1, OsCPS2~OsCyc2, gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein PBZ1 in response to cold stress and pathogen attack, Specifically, OsCPS1, which encodes an enzyme in the GA biosynthesis pathway, was down-regulated and OsCPS2 and OsCPS4, which encode enzymes in phytoalexin biosynthesis, were up-regulated in gid1
- GID1~OsGID1, SLR1~OsGAI, The suppressive function of the rice DELLA protein SLR1 is dependent on its transcriptional activation activity, This activity was suppressed by the GA-dependent GID1-SLR1 interaction, which may explain why GA responses are induced in the presence of GA
- GID1~OsGID1, SLR1~OsGAI, The suppressive function of the rice DELLA protein SLR1 is dependent on its transcriptional activation activity, Our results indicate that the N-terminal region of SLR1 has two roles in GA signaling: interaction with GID1 and transactivation activity
- GID1~OsGID1, SLR1~OsGAI, GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin, Moreover, GID1 bound to SLR1, a rice DELLA protein, in a GA-dependent manner in yeast cells
- OsGF14e~GF14e~GID2, GID1~OsGID1, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, GA perception by GID1 causes SLR1 protein degradation involving the F-box protein GID2; this triggers GA-associated responses such as shoot elongation and seed germination
- OsGF14e~GF14e~GID2, GID1~OsGID1, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, An exception is the GA-insensitive F-box mutant gid2, which shows milder dwarfism than mutants such as gid1 and cps even though it accumulates higher levels of SLR1
- OsGF14e~GF14e~GID2, GID1~OsGID1, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, The level of SLR1 protein in gid2 was decreased by loss of GID1 function or treatment with a GA biosynthesis inhibitor, and dwarfism was enhanced
- OsGF14e~GF14e~GID2, GID1~OsGID1, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, Conversely, overproduction of GID1 or treatment with GA(3) increased the SLR1 level in gid2 and reduced dwarfism
- OsGF14e~GF14e~GID2, GID1~OsGID1, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, These results indicate that derepression of SLR1 repressive activity can be accomplished by GA and GID1 alone and does not require F-box (GID2) function
- OsGF14e~GF14e~GID2, GID1~OsGID1, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, Evidence for GA signaling without GID2 was also provided by the expression behavior of GA-regulated genes such as GA-20oxidase1, GID1, and SLR1 in the gid2 mutant
- GID1~OsGID1, SLR1~OsGAI, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, GA perception by GID1 causes SLR1 protein degradation involving the F-box protein GID2; this triggers GA-associated responses such as shoot elongation and seed germination
- GID1~OsGID1, SLR1~OsGAI, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, An exception is the GA-insensitive F-box mutant gid2, which shows milder dwarfism than mutants such as gid1 and cps even though it accumulates higher levels of SLR1
- GID1~OsGID1, SLR1~OsGAI, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, The level of SLR1 protein in gid2 was decreased by loss of GID1 function or treatment with a GA biosynthesis inhibitor, and dwarfism was enhanced
- GID1~OsGID1, SLR1~OsGAI, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, Conversely, overproduction of GID1 or treatment with GA(3) increased the SLR1 level in gid2 and reduced dwarfism
- GID1~OsGID1, SLR1~OsGAI, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, These results indicate that derepression of SLR1 repressive activity can be accomplished by GA and GID1 alone and does not require F-box (GID2) function
- GID1~OsGID1, SLR1~OsGAI, Release of the repressive activity of rice DELLA protein SLR1 by gibberellin does not require SLR1 degradation in the gid2 mutant, Evidence for GA signaling without GID2 was also provided by the expression behavior of GA-regulated genes such as GA-20oxidase1, GID1, and SLR1 in the gid2 mutant
- GID1~OsGID1, SLR1~OsGAI, A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins, GID1(P99S) interacts with the rice DELLA protein SLENDER RICE1 (SLR1), even in the absence of GA
- GID1~OsGID1, SLR1~OsGAI, A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins, This suggests that the GID1(P99A) lid is at least partially closed, resulting in both GA-independent and GA-hypersensitive interactions with SLR1
- GID1~OsGID1, SLR1~OsGAI, A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins, Substitution of the loop region or a few amino acids of At GID1b with those of At GID1a diminished its GA-independent interaction with GAI while maintaining the GA-dependent interaction
Prev Next