- Information
- Symbol: OsBSK3
- MSU: LOC_Os04g58750
- RAPdb: Os04g0684200
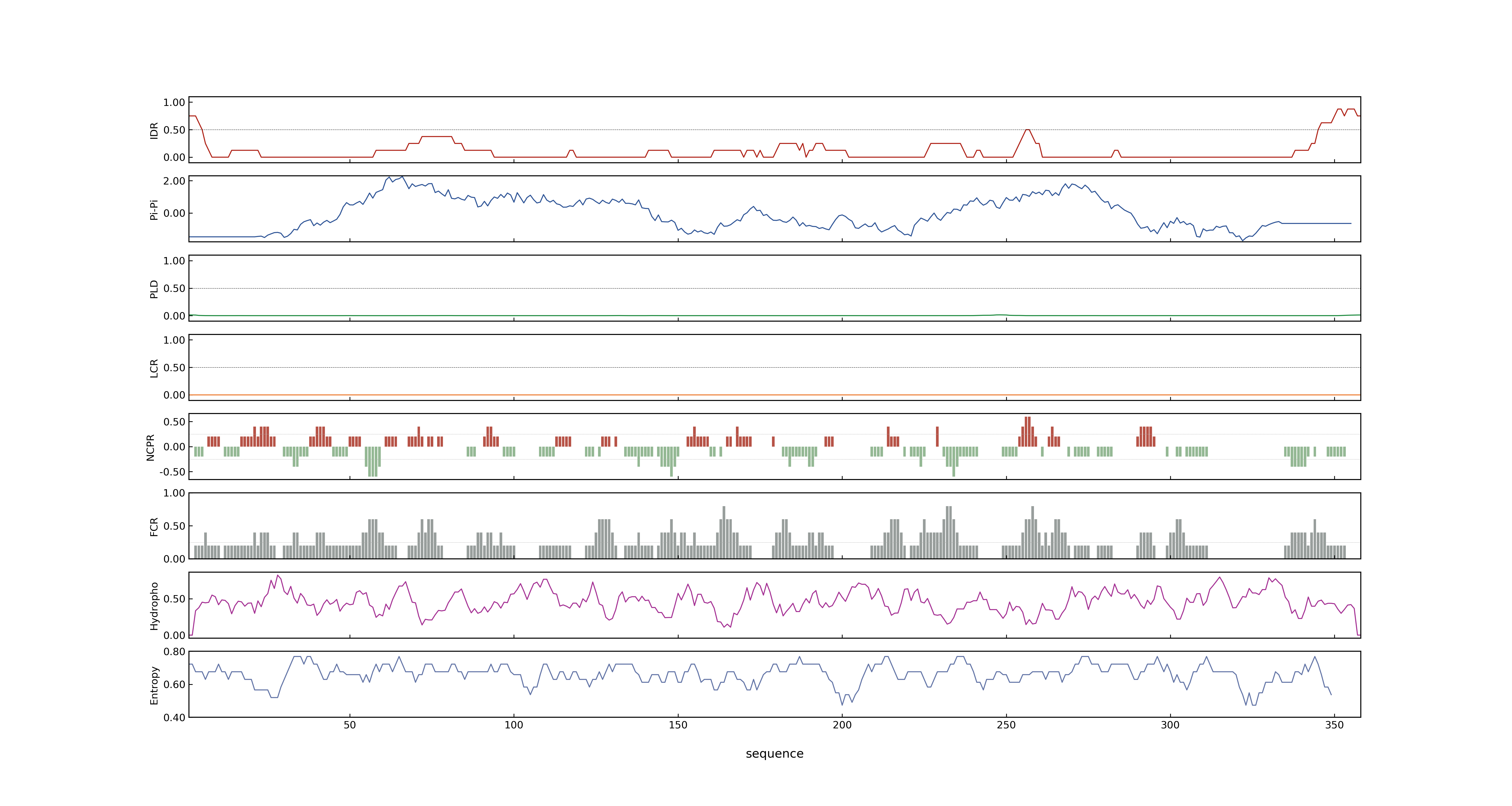
- PSP score
- LOC_Os04g58750.3: 0.0486
- LOC_Os04g58750.2: 0.0486
- LOC_Os04g58750.1: 0.0486
- LOC_Os04g58750.4: 0.0486
- PLAAC score
- LOC_Os04g58750.3: 0
- LOC_Os04g58750.2: 0
- LOC_Os04g58750.1: 0
- LOC_Os04g58750.4: 0
- pLDDT score
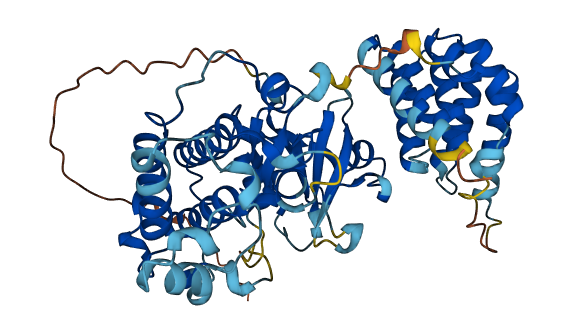
- 83.59
- Protein Structure from AlphaFold and UniProt
- MolPhase score
- LOC_Os04g58750.1: 0.05870716
- LOC_Os04g58750.2: 0.05870716
- LOC_Os04g58750.3: 0.05870716
- LOC_Os04g58750.4: 0.05870716
- MolPhase Result
- Publication
-
Genbank accession number
- Key message
- OsBRI1 activates BR signaling by preventing binding between the TPR and kinase domains of OsBSK3 via phosphorylation.
- Genetic studies revealed that OsBSK3 is a positive regulator of BR signaling in rice, while in vivo and in vitro assays demonstrated that OsBRI1 interacts directly with and phosphorylates OsBSK3
- Our results not only demonstrate that OsBSK3 plays a conserved role in regulating BR signaling in rice, but also provide insight into the molecular mechanism by which BSK family proteins are inhibited under basal conditions but switched on by the upstream receptor kinase BRI1
- The TPR domain of OsBSK3, which interacts directly with the protein’s kinase domain, serves as an autoinhibitory domain to prevent OsBSK3 from interacting with BSU1
- Phosphorylation of OsBSK3 by OsBRI1 disrupts the interaction between its TPR and kinase domains, thereby increasing the binding between OsBSK3’s kinase domain and BSU1
- Connection
- D61~OsBRI1, OsBSK3, OsBRI1 activates BR signaling by preventing binding between the TPR and kinase domains of OsBSK3 via phosphorylation., OsBRI1 activates BR signaling by preventing binding between the TPR and kinase domains of OsBSK3 via phosphorylation.
- D61~OsBRI1, OsBSK3, OsBRI1 activates BR signaling by preventing binding between the TPR and kinase domains of OsBSK3 via phosphorylation., Genetic studies revealed that OsBSK3 is a positive regulator of BR signaling in rice, while in vivo and in vitro assays demonstrated that OsBRI1 interacts directly with and phosphorylates OsBSK3
- D61~OsBRI1, OsBSK3, OsBRI1 activates BR signaling by preventing binding between the TPR and kinase domains of OsBSK3 via phosphorylation., Phosphorylation of OsBSK3 by OsBRI1 disrupts the interaction between its TPR and kinase domains, thereby increasing the binding between OsBSK3’s kinase domain and BSU1
Prev Next