- Information
- Symbol: OsHIR1
- MSU: LOC_Os08g30790
- RAPdb: Os08g0398400
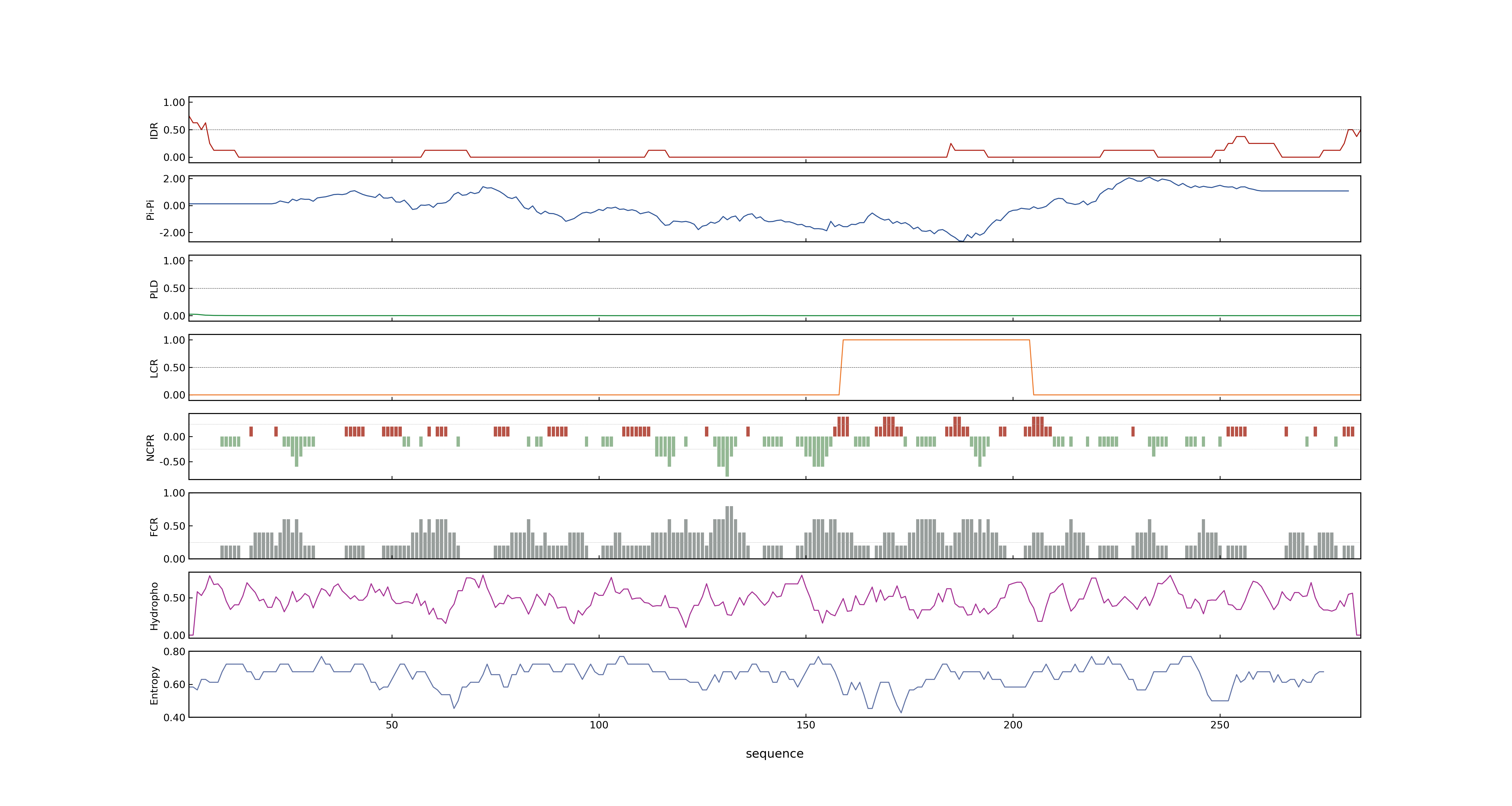
- PSP score
- LOC_Os08g30790.3: 0.0142
- LOC_Os08g30790.1: 0.0142
- LOC_Os08g30790.2: 0.0142
- PLAAC score
- LOC_Os08g30790.3: 0
- LOC_Os08g30790.1: 0
- LOC_Os08g30790.2: 0
- pLDDT score
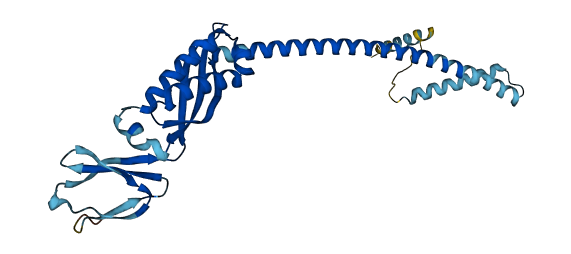
- 86.7
- Protein Structure from AlphaFold and UniProt
- MolPhase score
- LOC_Os08g30790.1: 0.01242537
- LOC_Os08g30790.2: 0.01242537
- LOC_Os08g30790.3: 0.01242537
- MolPhase Result
- Publication
- Rice hypersensitive induced reaction protein 1 OsHIR1 associates with plasma membrane and triggers hypersensitive cell death, 2010, BMC Plant Biol.
- A novel simple extracellular leucine-rich repeat eLRR domain protein from rice OsLRR1 enters the endosomal pathway and interacts with the hypersensitive-induced reaction protein 1 OsHIR1, 2009, Plant Cell Environ.
- Genbank accession number
- Key message
- Here we show that OsHIR1 triggers hypersensitive cell death and its localization to the plasma membrane is enhanced by OsLRR1
- Rice hypersensitive induced reaction protein 1 (OsHIR1) associates with plasma membrane and triggers hypersensitive cell death
- Connection
- OsHIR1, OsLRR1, A novel simple extracellular leucine-rich repeat eLRR domain protein from rice OsLRR1 enters the endosomal pathway and interacts with the hypersensitive-induced reaction protein 1 OsHIR1, Yeast two-hybrid and in vitro pull-down assays show that OsLRR1 interacts with the rice hypersensitive-induced response protein 1 (OsHIR1) which is localized on plasma membrane
- OsHIR1, OsLRR1, A novel simple extracellular leucine-rich repeat eLRR domain protein from rice OsLRR1 enters the endosomal pathway and interacts with the hypersensitive-induced reaction protein 1 OsHIR1, A novel simple extracellular leucine-rich repeat (eLRR) domain protein from rice (OsLRR1) enters the endosomal pathway and interacts with the hypersensitive-induced reaction protein 1 (OsHIR1)
- OsHIR1, OsLRR1, A novel simple extracellular leucine-rich repeat eLRR domain protein from rice OsLRR1 enters the endosomal pathway and interacts with the hypersensitive-induced reaction protein 1 OsHIR1, The interaction between LRR1 and HIR1 homologs was shown to be highly conserved among different plant species, suggesting a close functional relationship between the two proteins
Prev Next