- Information
- Symbol: PHYA,OsPhyA
- MSU: LOC_Os03g51030
- RAPdb: Os03g0719800
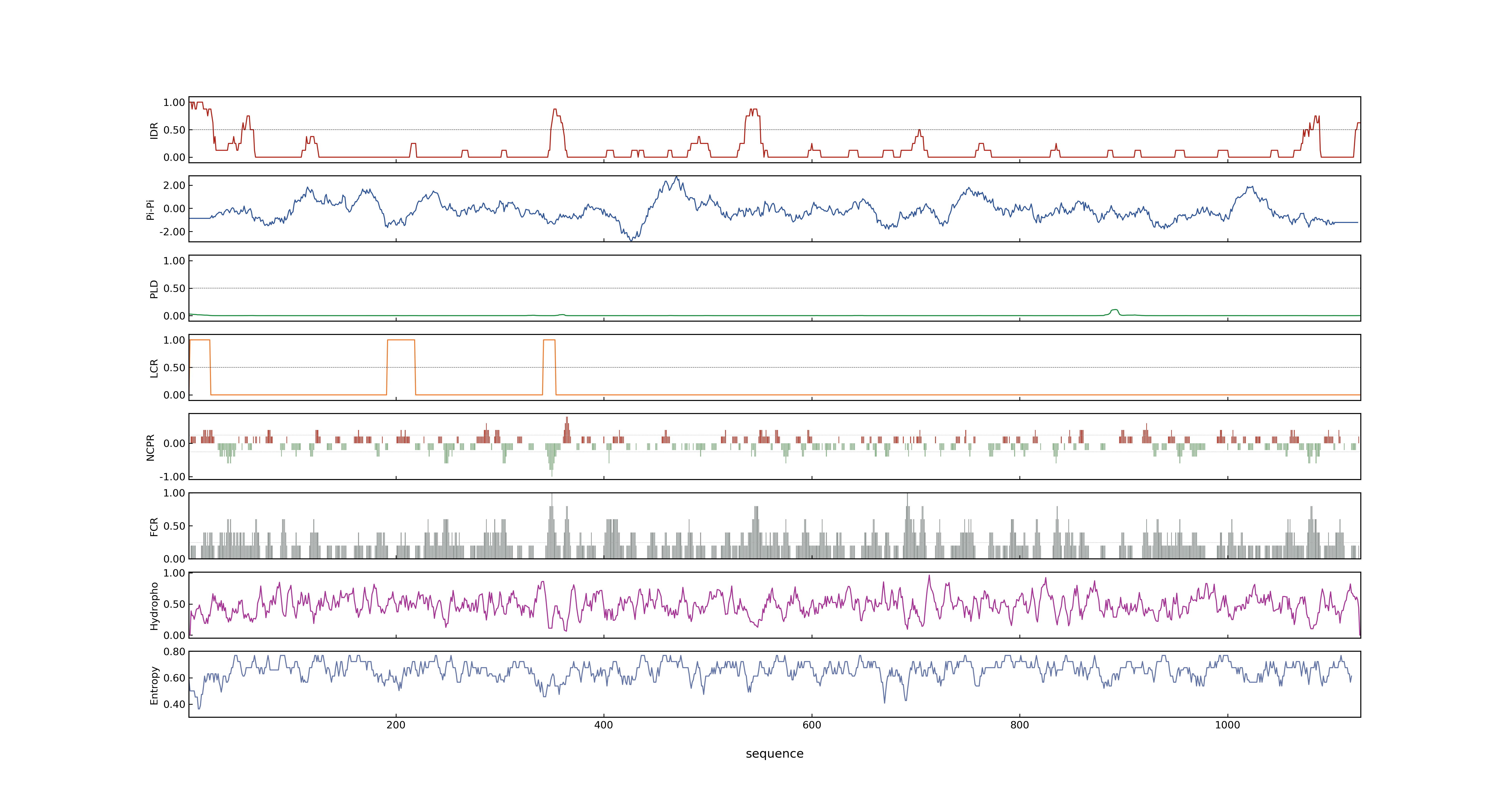
- PSP score
- LOC_Os03g51030.3: 0.0678
- LOC_Os03g51030.1: 0.0678
- LOC_Os03g51030.2: 0.0678
- PLAAC score
- LOC_Os03g51030.3: 0
- LOC_Os03g51030.1: 0
- LOC_Os03g51030.2: 0
(Zheng et al., New Phytol, 2019, 224:306-320)
- pLDDT score
- 77.44
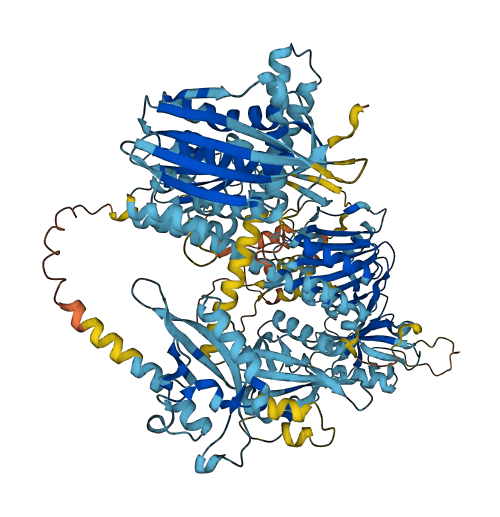
- Protein Structure from AlphaFold and UniProt
- MolPhase score
- LOC_Os03g51030.1: 0.99664598
- LOC_Os03g51030.2: 0.99664598
- LOC_Os03g51030.3: 0.99664598
- MolPhase Result
- Publication
- Isolation and characterization of rice phytochrome A mutants, 2001, Plant Cell.
- Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice, 2011, Plant Physiol.
- The multiple contributions of phytochromes to the control of internode elongation in rice, 2011, Plant Physiol.
- Serine-to-alanine substitutions at the amino-terminal region of phytochrome A result in an increase in biological activity, 1992, Genes Dev.
- Cryptochrome and phytochrome cooperatively but independently reduce active gibberellin content in rice seedlings under light irradiation, 2012, Plant Cell Physiol.
- Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling, 2008, Plant Cell Environ.
- The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter, 1999, The Plant Journal.
- Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice, 2009, Proc Natl Acad Sci U S A.
- Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, 2005, Plant Cell.
- Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, 2009, J Biosci Bioeng.
- OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B., 2016, Plant Mol Biol.
- Genbank accession number
- Key message
- ACO1 expression was controlled mainly by phyA and phyB, and a histochemical analysis showed that ACO1 expression was localized to the basal parts of leaf sheaths of phyAphyBphyC seedlings, similar to mature wild-type plants at the heading stage, when internode elongation was greatly promoted
- Rice is a short-day plant, and we found that mutation in either phyB or phyC caused moderate early flowering under the long-day photoperiod, while monogenic phyA mutation had little effect on the flowering time
- The phyA mutation, however, in combination with phyB or phyC mutation caused dramatic early flowering
- The seedlings of phyA mutants grown in continuous far-red light showed essentially the same phenotype as dark-grown seedlings, indicating the insensitivity of phyA mutants to far-red light
- The etiolated seedlings of phyA mutants also were insensitive to a pulse of far-red light or very low fluence red light
- Interestingly, continuous far-red light induced the expression of CAB and RBCS genes in rice phyA seedlings, suggesting the existence of a photoreceptor(s) other than phyA that can perceive continuous far-red light in the etiolated seedlings
- However, hypocotyl elongation experiments revealed that transgenic seedlings expressing S/A phyA showed a higher amplitude of the red light response with respect to the inhibition of hypocotyl elongation
- In addition, the transcription levels of several ethylene- or gibberellin (GA)-related genes were changed in phyAphyBphyC mutants, and measurement of the plant hormone levels indicated low ethylene production and bioactive GA levels in the phyAphyBphyC mutants
- The metabolite profiles indicated high accumulation of amino acids, organic acids, sugars, sugar phosphates, and nucleotides in the leaf blades of phyA phyB phyC triple mutants, especially in the young leaves, compared with those in the WT
- A gene for 1-aminocyclopropane-1-carboxylate oxidase (ACO1), which is an ethylene biosynthesis gene contributing to internode elongation, was up-regulated in phyAphyBphyC seedlings
- We demonstrate that ethylene induced internode elongation and ACO1 expression in phyAphyBphyC seedlings but not in the wild type and that the presence of bioactive GAs was necessary for these effects
- Moreover, phyB and phyA can affect Ghd7 activity and Early heading date1 (a floral inducer) activity in the network, respectively
- Although phyAphyBphyC phytochrome-null mutants in rice (Oryza sativa) have morphological changes and exhibit internode elongation, even as seedlings, it is unknown how phytochromes contribute to the control of internode elongation
- Transgenic tobacco plants expressing either wild-type or S/A phyA showed similar phenotypic alterations, including dwarfism and dark-green leaves
- Distinct metabolic profiles between phyA phyB phyC triple mutants and the wild type (WT), as well as those between young and mature leaf blades, could be clearly observed by principal component analysis (PCA)
- OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B.
- These results indicated that OsPhyA influences flowering time mainly by affecting the expression of OsGI under SD and Ghd7 under LD when phytochrome B is absent
- We also demonstrated that far-red light delays flowering time via both OsPhyA and OsPhyB
- Connection
- PHYA~OsPhyA, PHYC, Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice, Our results show that either phyA alone or a genetic combination of phyB and phyC can induce Ghd7 mRNA, whereas phyB alone causes some reduction in levels of Ghd7 mRNA
- Ghd7, PHYA~OsPhyA, Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice, Our results show that either phyA alone or a genetic combination of phyB and phyC can induce Ghd7 mRNA, whereas phyB alone causes some reduction in levels of Ghd7 mRNA
- Ghd7, PHYA~OsPhyA, Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice, Moreover, phyB and phyA can affect Ghd7 activity and Early heading date1 (a floral inducer) activity in the network, respectively
- PHYA~OsPhyA, PHYB~OsphyB, Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice, Our results show that either phyA alone or a genetic combination of phyB and phyC can induce Ghd7 mRNA, whereas phyB alone causes some reduction in levels of Ghd7 mRNA
- PHYA~OsPhyA, PHYB~OsphyB, Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice, Moreover, phyB and phyA can affect Ghd7 activity and Early heading date1 (a floral inducer) activity in the network, respectively
- OsACO1, PHYA~OsPhyA, The multiple contributions of phytochromes to the control of internode elongation in rice, A gene for 1-aminocyclopropane-1-carboxylate oxidase (ACO1), which is an ethylene biosynthesis gene contributing to internode elongation, was up-regulated in phyAphyBphyC seedlings
- OsACO1, PHYA~OsPhyA, The multiple contributions of phytochromes to the control of internode elongation in rice, ACO1 expression was controlled mainly by phyA and phyB, and a histochemical analysis showed that ACO1 expression was localized to the basal parts of leaf sheaths of phyAphyBphyC seedlings, similar to mature wild-type plants at the heading stage, when internode elongation was greatly promoted
- OsACO1, PHYA~OsPhyA, The multiple contributions of phytochromes to the control of internode elongation in rice, We demonstrate that ethylene induced internode elongation and ACO1 expression in phyAphyBphyC seedlings but not in the wild type and that the presence of bioactive GAs was necessary for these effects
- PHYA~OsPhyA, PHYC, The multiple contributions of phytochromes to the control of internode elongation in rice, Although phyAphyBphyC phytochrome-null mutants in rice (Oryza sativa) have morphological changes and exhibit internode elongation, even as seedlings, it is unknown how phytochromes contribute to the control of internode elongation
- PHYA~OsPhyA, PHYC, The multiple contributions of phytochromes to the control of internode elongation in rice, A gene for 1-aminocyclopropane-1-carboxylate oxidase (ACO1), which is an ethylene biosynthesis gene contributing to internode elongation, was up-regulated in phyAphyBphyC seedlings
- PHYA~OsPhyA, PHYC, The multiple contributions of phytochromes to the control of internode elongation in rice, ACO1 expression was controlled mainly by phyA and phyB, and a histochemical analysis showed that ACO1 expression was localized to the basal parts of leaf sheaths of phyAphyBphyC seedlings, similar to mature wild-type plants at the heading stage, when internode elongation was greatly promoted
- PHYA~OsPhyA, PHYC, The multiple contributions of phytochromes to the control of internode elongation in rice, In addition, the transcription levels of several ethylene- or gibberellin (GA)-related genes were changed in phyAphyBphyC mutants, and measurement of the plant hormone levels indicated low ethylene production and bioactive GA levels in the phyAphyBphyC mutants
- PHYA~OsPhyA, PHYC, The multiple contributions of phytochromes to the control of internode elongation in rice, We demonstrate that ethylene induced internode elongation and ACO1 expression in phyAphyBphyC seedlings but not in the wild type and that the presence of bioactive GAs was necessary for these effects
- PHYA~OsPhyA, PHYB~OsphyB, The multiple contributions of phytochromes to the control of internode elongation in rice, Although phyAphyBphyC phytochrome-null mutants in rice (Oryza sativa) have morphological changes and exhibit internode elongation, even as seedlings, it is unknown how phytochromes contribute to the control of internode elongation
- PHYA~OsPhyA, PHYB~OsphyB, The multiple contributions of phytochromes to the control of internode elongation in rice, A gene for 1-aminocyclopropane-1-carboxylate oxidase (ACO1), which is an ethylene biosynthesis gene contributing to internode elongation, was up-regulated in phyAphyBphyC seedlings
- PHYA~OsPhyA, PHYB~OsphyB, The multiple contributions of phytochromes to the control of internode elongation in rice, ACO1 expression was controlled mainly by phyA and phyB, and a histochemical analysis showed that ACO1 expression was localized to the basal parts of leaf sheaths of phyAphyBphyC seedlings, similar to mature wild-type plants at the heading stage, when internode elongation was greatly promoted
- PHYA~OsPhyA, PHYB~OsphyB, The multiple contributions of phytochromes to the control of internode elongation in rice, In addition, the transcription levels of several ethylene- or gibberellin (GA)-related genes were changed in phyAphyBphyC mutants, and measurement of the plant hormone levels indicated low ethylene production and bioactive GA levels in the phyAphyBphyC mutants
- PHYA~OsPhyA, PHYB~OsphyB, The multiple contributions of phytochromes to the control of internode elongation in rice, We demonstrate that ethylene induced internode elongation and ACO1 expression in phyAphyBphyC seedlings but not in the wild type and that the presence of bioactive GAs was necessary for these effects
- PHYA~OsPhyA, PHYB~OsphyB, Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling, The analysis of OsJar1 expression in phytochrome (phy) mutants revealed that phytochrome A (phyA) and phytochrome B (phyB) act redundantly to induce this gene by red light, presumably
- OsJar1~OsGH3.5~OsGH3-5, PHYA~OsPhyA, Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling, The analysis of OsJar1 expression in phytochrome (phy) mutants revealed that phytochrome A (phyA) and phytochrome B (phyB) act redundantly to induce this gene by red light, presumably
- OsJar1~OsGH3.5~OsGH3-5, PHYA~OsPhyA, Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling, Unexpectedly, blue light-induced expression of OsJar1 gene was impaired in phyA-deficient mutants, indicating the involvement of phyA in the blue light signalling
- GT-2, PHYA~OsPhyA, The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter, The DNA-binding proteins PF1 and GT-2 are factors that bind to different functionally defined, positively acting cis-elements in the PHYA genes of oat and rice, respectively
- GT-2, PHYA~OsPhyA, The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter, The evidence indicates therefore that PF1 and GT-2 do not perform functionally equivalent roles in positively regulating oat and rice PHYA gene expression
- GT-2, PHYA~OsPhyA, The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter, The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter
- PF1, PHYA~OsPhyA, The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter, The DNA-binding proteins PF1 and GT-2 are factors that bind to different functionally defined, positively acting cis-elements in the PHYA genes of oat and rice, respectively
- PF1, PHYA~OsPhyA, The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter, The evidence indicates therefore that PF1 and GT-2 do not perform functionally equivalent roles in positively regulating oat and rice PHYA gene expression
- PF1, PHYA~OsPhyA, The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter, The HMG-I/Y protein PF1 stimulates binding of the transcriptional activator GT-2 to the PHYA gene promoter
- PHYA~OsPhyA, PHYB~OsphyB, Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice, To directly address this hypothesis, a phytochrome triple mutant (phyAphyBphyC) was generated in rice (Oryza sativa L
- PHYA~OsPhyA, PHYB~OsphyB, Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice, Since rice only has three phytochrome genes (PHYA, PHYB and PHYC), this mutant is completely lacking any phytochrome
- PHYA~OsPhyA, PHYC, Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice, To directly address this hypothesis, a phytochrome triple mutant (phyAphyBphyC) was generated in rice (Oryza sativa L
- PHYA~OsPhyA, PHYC, Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice, Since rice only has three phytochrome genes (PHYA, PHYB and PHYC), this mutant is completely lacking any phytochrome
- PHYA~OsPhyA, PHYB~OsphyB, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, The responses to Rc were completely canceled in phyA phyB double mutants
- PHYA~OsPhyA, PHYB~OsphyB, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, These results indicate that phyA and phyB act in a highly redundant manner to control deetiolation under Rc
- PHYA~OsPhyA, PHYB~OsphyB, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, Interestingly, the phyB phyC double mutant displayed clear R/FR reversibility in the pulse irradiation experiments, indicating that both phyA and phyB can mediate the low-fluence response for gene expression
- PHYA~OsPhyA, PHYB~OsphyB, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, Rice is a short-day plant, and we found that mutation in either phyB or phyC caused moderate early flowering under the long-day photoperiod, while monogenic phyA mutation had little effect on the flowering time
- PHYA~OsPhyA, PHYB~OsphyB, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, The phyA mutation, however, in combination with phyB or phyC mutation caused dramatic early flowering
- PHYA~OsPhyA, PHYC, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, Under continuous far-red light (FRc), phyA mutants showed partially impaired deetiolation, and phyA phyC double mutants showed no significant residual phytochrome responses, indicating that not only phyA but also phyC is involved in the photoperception of FRc in rice
- PHYA~OsPhyA, PHYC, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, Interestingly, the phyB phyC double mutant displayed clear R/FR reversibility in the pulse irradiation experiments, indicating that both phyA and phyB can mediate the low-fluence response for gene expression
- PHYA~OsPhyA, PHYC, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, Rice is a short-day plant, and we found that mutation in either phyB or phyC caused moderate early flowering under the long-day photoperiod, while monogenic phyA mutation had little effect on the flowering time
- PHYA~OsPhyA, PHYC, Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice, The phyA mutation, however, in combination with phyB or phyC mutation caused dramatic early flowering
- PHYA~OsPhyA, PHYB~OsphyB, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, Rice (Oryza sativa) possesses three phytochromes, phyA, phyB, and phyC
- PHYA~OsPhyA, PHYB~OsphyB, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, In the present study, non-targeted metabolite analysis by gas chromatography time-of-flight mass spectrometry (GC/TOF-MS) and targeted metabolite analysis by capillary electrophoresis electrospray ionization mass spectrometry (CE/ESI-MS) were employed to investigate metabolic changes in rice phyA phyB phyC triple mutants
- PHYA~OsPhyA, PHYB~OsphyB, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, Distinct metabolic profiles between phyA phyB phyC triple mutants and the wild type (WT), as well as those between young and mature leaf blades, could be clearly observed by principal component analysis (PCA)
- PHYA~OsPhyA, PHYB~OsphyB, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, The metabolite profiles indicated high accumulation of amino acids, organic acids, sugars, sugar phosphates, and nucleotides in the leaf blades of phyA phyB phyC triple mutants, especially in the young leaves, compared with those in the WT
- PHYA~OsPhyA, PHYB~OsphyB, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, 5-fold), was observed in young leaves of phyA phyB phyC triple mutants
- PHYA~OsPhyA, PHYB~OsphyB, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, These metabolic phenotypes suggest that sugar metabolism, carbon partitioning, sugar transport, or some combination of these is impaired in the phyA phyB phyC triple mutants, and conversely, that phytochromes have crucial roles in sugar metabolism
- PHYA~OsPhyA, PHYB~OsphyB, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves
- PHYA~OsPhyA, PHYC, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, Rice (Oryza sativa) possesses three phytochromes, phyA, phyB, and phyC
- PHYA~OsPhyA, PHYC, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, In the present study, non-targeted metabolite analysis by gas chromatography time-of-flight mass spectrometry (GC/TOF-MS) and targeted metabolite analysis by capillary electrophoresis electrospray ionization mass spectrometry (CE/ESI-MS) were employed to investigate metabolic changes in rice phyA phyB phyC triple mutants
- PHYA~OsPhyA, PHYC, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, Distinct metabolic profiles between phyA phyB phyC triple mutants and the wild type (WT), as well as those between young and mature leaf blades, could be clearly observed by principal component analysis (PCA)
- PHYA~OsPhyA, PHYC, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, The metabolite profiles indicated high accumulation of amino acids, organic acids, sugars, sugar phosphates, and nucleotides in the leaf blades of phyA phyB phyC triple mutants, especially in the young leaves, compared with those in the WT
- PHYA~OsPhyA, PHYC, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, 5-fold), was observed in young leaves of phyA phyB phyC triple mutants
- PHYA~OsPhyA, PHYC, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, These metabolic phenotypes suggest that sugar metabolism, carbon partitioning, sugar transport, or some combination of these is impaired in the phyA phyB phyC triple mutants, and conversely, that phytochromes have crucial roles in sugar metabolism
- PHYA~OsPhyA, PHYC, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves, Comprehensive metabolite profiling of phyA phyB phyC triple mutants to reveal their associated metabolic phenotype in rice leaves
- Ghd7, PHYA~OsPhyA, OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B., OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B.
- Ghd7, PHYA~OsPhyA, OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B., These results indicated that OsPhyA influences flowering time mainly by affecting the expression of OsGI under SD and Ghd7 under LD when phytochrome B is absent
- OsGI, PHYA~OsPhyA, OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B., OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B.
- OsGI, PHYA~OsPhyA, OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B., These results indicated that OsPhyA influences flowering time mainly by affecting the expression of OsGI under SD and Ghd7 under LD when phytochrome B is absent
- PHYA~OsPhyA, PHYB~OsphyB, OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B., We investigated the role of OsPhyA by comparing the osphyA osphyB double mutant to an osphyB single mutant
- PHYA~OsPhyA, PHYB~OsphyB, OsPhyA modulates rice flowering time mainly through OsGI under short days and Ghd7 under long days in the absence of phytochrome B., We also demonstrated that far-red light delays flowering time via both OsPhyA and OsPhyB
- OsCOL13, PHYA~OsPhyA, A CONSTANS-like transcriptional activator, OsCOL13, functions as a negative regulator of flowering downstream of OsphyB and upstream of Ehd1 in rice., In addition, the transcriptional level of OsCOL13 significantly decreased in the osphyb mutant, but remained unchanged in the osphya and osphyc mutants