- Information
- Symbol: RH3
- MSU: LOC_Os01g32380
- RAPdb: Os01g0508100
- PSP score
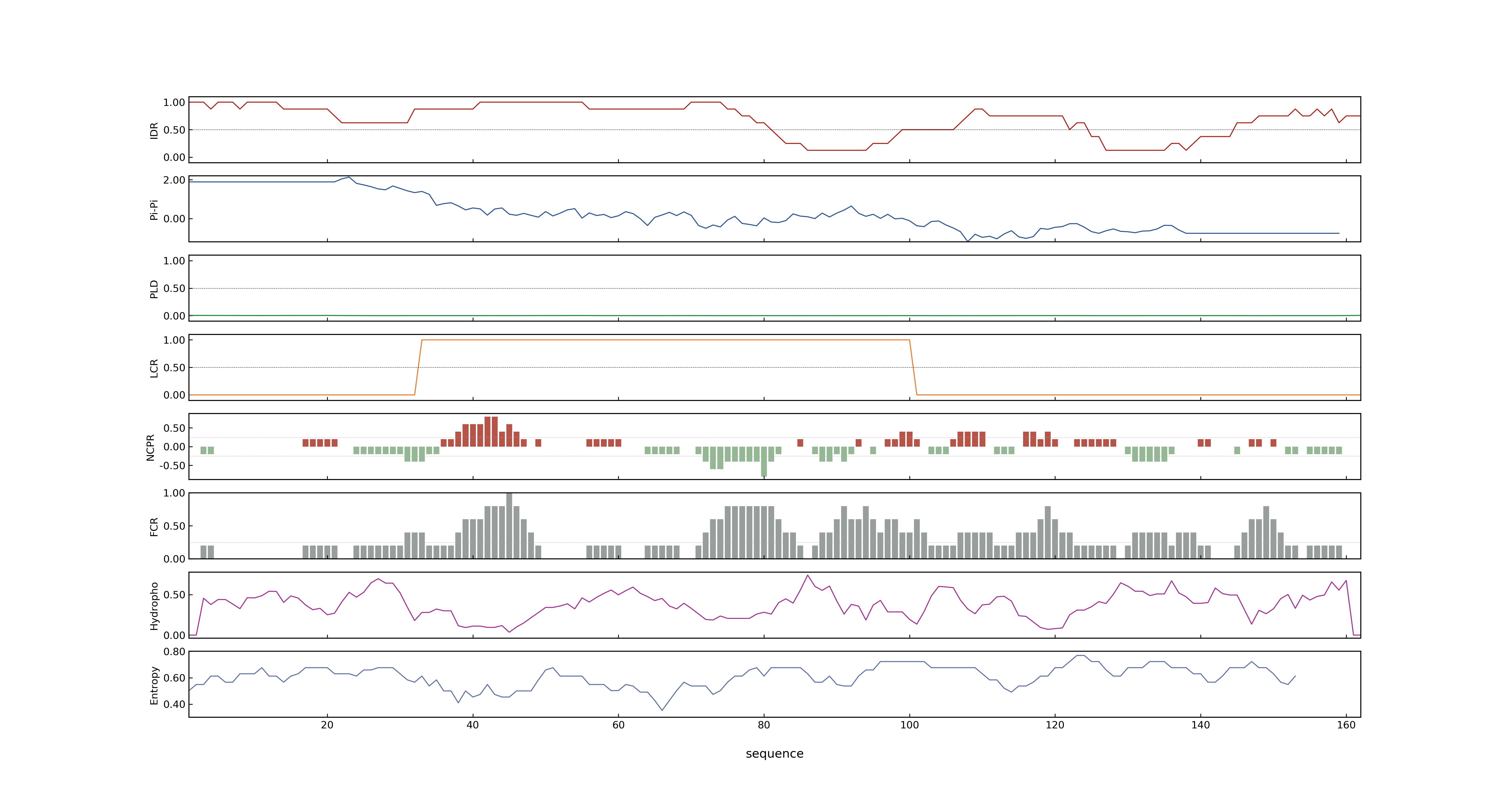
- LOC_Os01g32380.1: 0.2278
- PLAAC score
- LOC_Os01g32380.1: 0
- pLDDT score
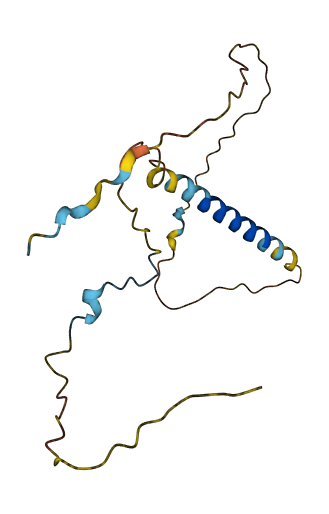
- 62.27
- Protein Structure from AlphaFold and UniProt
- MolPhase score
- LOC_Os01g32380.1: 0.99021446
- MolPhase Result
- Publication
-
Genbank accession number
-
Key message
- Connection
- NRR~CRCT, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, We identified three NRR homologues (RH1, RH2, and RH3)
- NRR~CRCT, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, NRR, RH1, RH2, and RH3 share sequence similarity in a region beyond the previously identified NPR1-interacting domain
- NRR~CRCT, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, RH2 carries a deviation (amino acids AV) in this region as compared to consensus sequences (amino acids ED) among NRR, RH1, and RH3
- OsNPR1~NH1, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, RH1 and RH3, but not RH2, also effectively repress NH1-mediated transcriptional activation
- OsNPR1~NH1, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, NRR, RH1, RH2, and RH3 share sequence similarity in a region beyond the previously identified NPR1-interacting domain
- RH2, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, We identified three NRR homologues (RH1, RH2, and RH3)
- RH2, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, RH1 and RH3, but not RH2, also effectively repress NH1-mediated transcriptional activation
- RH2, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, NRR, RH1, RH2, and RH3 share sequence similarity in a region beyond the previously identified NPR1-interacting domain
- RH2, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, RH2 carries a deviation (amino acids AV) in this region as compared to consensus sequences (amino acids ED) among NRR, RH1, and RH3
- RH1, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, We identified three NRR homologues (RH1, RH2, and RH3)
- RH1, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, RH1 and RH3, but not RH2, also effectively repress NH1-mediated transcriptional activation
- RH1, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, NRR, RH1, RH2, and RH3 share sequence similarity in a region beyond the previously identified NPR1-interacting domain
- RH1, RH3, A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation, RH2 carries a deviation (amino acids AV) in this region as compared to consensus sequences (amino acids ED) among NRR, RH1, and RH3
Prev Next